Have you ever considered the importance of bone health for longevity and athletic performance? In our latest X-Change, written by Professor Craig Sale, we'll take a look at why bone health is so important to the athlete and their overall health.
Read the full X-Change article here or download the pdf via the button below
Key Points
- Athletes should pay attention to their bone health, both in terms of the risk of bone injury during their competitive years, for the longer-term exacerbation of the ageing- related losses of bone that inevitably occur.
- Stress fractures are relatively common injuries in many sports, particularly where the bone is subjected to high loading. It is difficult to provide accurate data on stress fracture injury incidence, since this is influenced by so many different factors, including, for example: age, race, type of sport, specialisation and location.
- Stress fractures are serious injuries and can result in significant time out of training and competition. For example, Ranson et al. (2010) reported that the mean amount of time lost per stress fracture in elite cricket fast bowlers was 169 days.
- Bone is a nutritionally modified tissue and, in general, the nutritional guidance given to an athlete for bone health is not that different to the guidance provided for the general population. Having said that, it is still unclear what the optimal intakes of these nutrients should be to support bone health of the athlete during periods of intense training and competition.
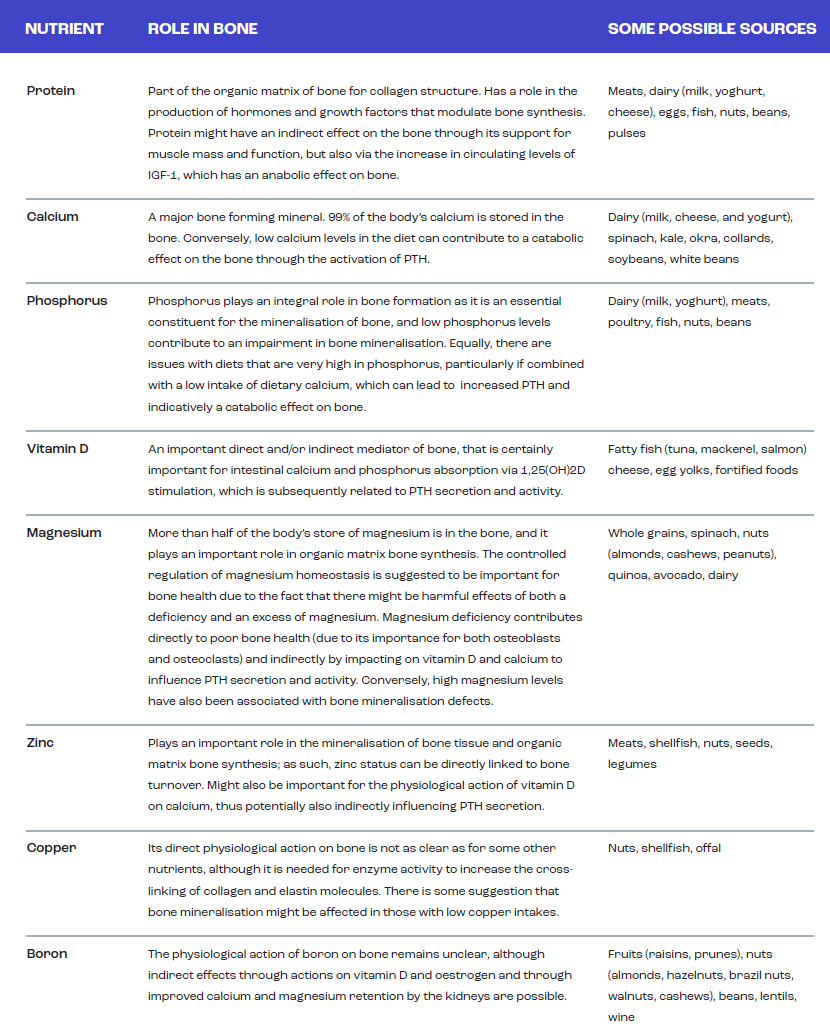
- The key nutrients to support bone health include protein, calcium, phosphorus, vitamin D, magnesium, zinc, copper, boron, manganese, potassium, iron, vitamin K, vitamin C, vitamin A, the B vitamins and silica.
- Some nutrients, particularly calcium and vitamin D, have also been suggested to be important in reducing the risk of stress fracture injury, although the links between dietary intake and stress fracture injury require further investigation.
- Further research is required into the effects of specific dietary practices now more commonly followed by athletes, such as plant based and ketogenic diets, on bone health and stress fracture injury risk.
- Some of the issues that might be of more specific relevance to the athlete include energy availability, low carbohydrate availability, protein intake, vitamin D intake and dermal calcium losses.
- More research related to the bone health of specific types of athletes is still needed.
Introduction
Bone health is of importance to an individual’s overall health and is a key component in the maintenance of quality of life and health span. Healthy bones provide an individual with a frame upon which to maintain mobility and protect the body from injury, although healthy bones are also required for blood cell production and to support mineral homeostasis. Furthermore, it is becoming clearer that the bone might also play a role in the control of energy metabolism.
Bone mineral density (BMD, or bone mass) changes across the lifespan with bone accrual progressing throughout childhood, adolescence and into early adulthood; around 90% of an individual’s peak BMD is achieved by the time that they are 20 years old (Figure 1). In general, BMD stabilises during early middle age before starting to decline when an individual reaches 40-50 years old, before accelerating in some, leading to bone health related issues such as osteopenia and osteoporosis. In addition to the changes in BMD that occur across the lifespan, there are also some sex specific differences that occur, with males generally achieving a higher peak BMD than females. Females also experience a more rapid loss of bone tissue with the menopause due to the removal of the protective effects of oestrogen on bone. These alterations in BMD are often also accompanied by a decrease in bone strength, osteocyte death, deterioration of type I collagen and adipogenesis at the expense of osteogenesis (Santos et al., 2017).
The gain and loss of bone tissue occurs because of alterations in the balance between bone resorption (loss) and bone formation (gain), with the three main cell types in bone (i.e., osteoblasts for bone formation, osteoclasts for bone resorption and osteocytes contributing to activation processes) being primarily responsible for these processes. The coordinated action of these cells influences bone modelling and remodelling (Figure 2). Bone modelling relates primarily to the processes that occur with the growth of bone during childhood, adolescence and early adulthood.
Bone remodelling more refers to the process that primarily occurs during adulthood to remove old and damaged bone to maintain the integrity and strength of the skeleton and to allow the bone to respond to the strains placed upon it from, for example, mechanical loading.
Bone modelling and remodelling
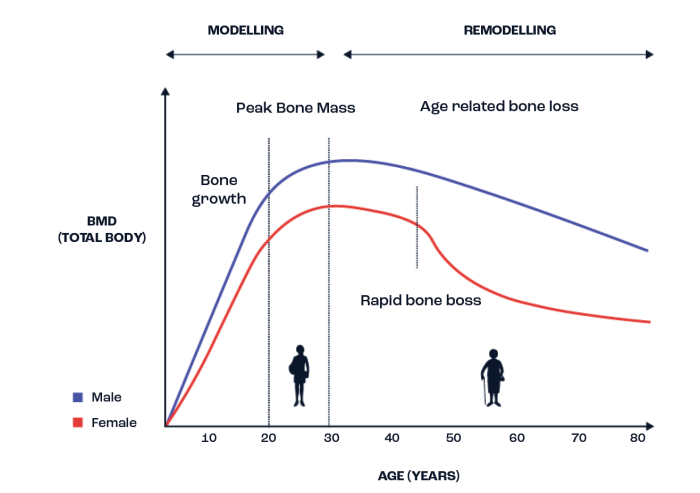
Figure 1. A depiction of how bone mineral density (BMD) typically changes across the lifespan in both males and females. Adapted from Hendrickx et al. (2015) and Santos et al. (2017).
Several non-modifiable and modifiable factors have been suggested to influence bone strengthening or weakening. Some of the non-modifiable factors include age, sex, race, and genetics, although there is little that can be done interventionally to influence their effects on bone. Of more interest from an interventional perspective are the modifiable factors, with perhaps the most influential of
these being mechanical loading. Other lifestyle factors, which primarily include diet and nutrition, smoking and sleep are also important and each of these factors can interact with various hormonal responses (for example with parathyroid hormone or oestrogen) to positively and/or negatively influence bone tissue.
Bone remodelling cycle
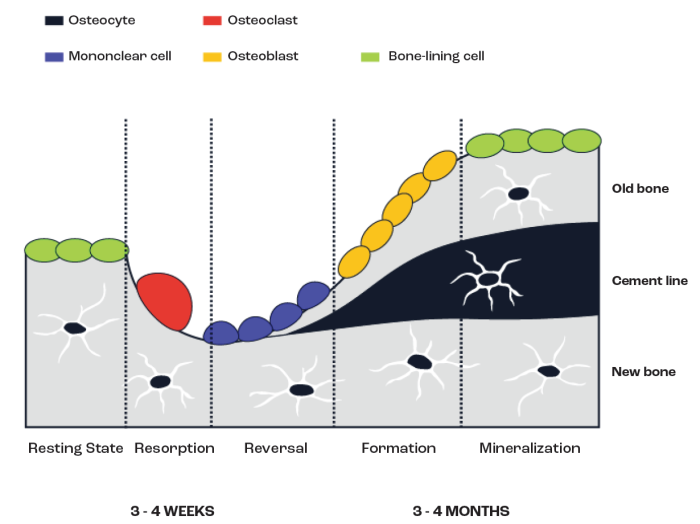
Figure 2. Illustration of the key factors associated with bone (re)modelling.
Stress fractures are the most common bone injuries suffered by athletes and they occur relatively regularly in many sports being caused by the rhythmic and repeated application of mechanical loading in a sub-threshold manner (McBryde, 1985). Since they are overuse injuries, high-volume, high-intensity training, where the athlete is body weight loaded, significantly increases the risk of stress fractures (Fredericson et al., 2007). These injuries can occur anywhere, although mainly (but not exclusively) in highly loaded bones. Therefore, the type of sport or activity has a significant influence on the location of the stress fracture. For example, tibial stress fractures are more common in distance runners, tarsal/metatarsal stress fractures are more common in football and basketball players, vertebral stress fractures are more common in cricket players and rib stress fractures are more common in rowers (Figure 3). It is important to note that as a result of stress fractures there is a likelihood of missed competitions and/or a significant amount of lost training time (Ranson et al., 2010). The pathophysiology of stress fracture injuries is complex and not completely understood, but it is likely that, at least for some athletes, dietary/nutritional inadequacies could increase the risk of their development.
Bone stress injuries
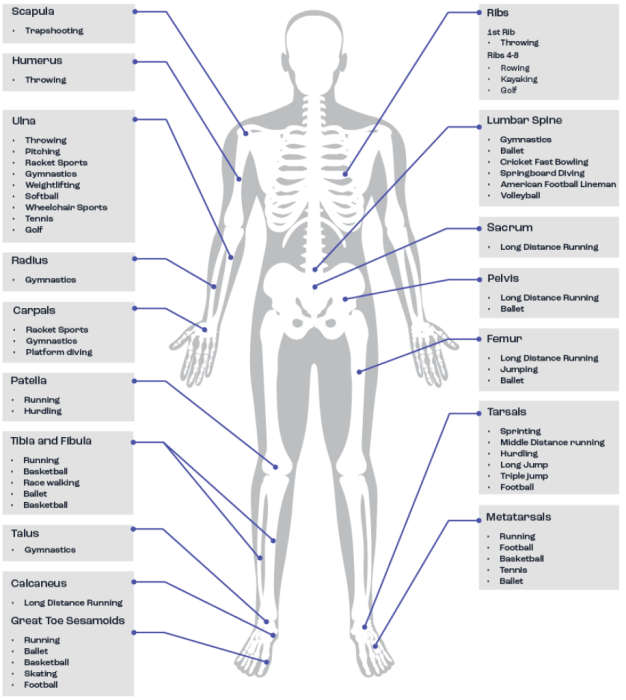
Figure 3. Common anatomical sites for bone stress injuries. Adapted from Hoenig et al. (2022).
In addition to the risk of injury, there is, at least for some sports, a longer-term risk to bone health, whereby the avoidance of osteopenia and osteoporosis becomes a consideration; particularly when bone mass is already recorded as being low during early adulthood. In addition to the importance of bone mass, the strength of the bone (which defines the bone’s ability to resist the strain placed upon it) should also be of primary concern for the athlete, both during and after their competitive years.
There are significant consequences of poor bone health in later life, particularly in relation to osteoporotic fracture, given that one fifth of individuals requiring hospitalisation for fragility fractures die within 6 months (National Institute for Health and Clinical Excellence, 2012).
As such, adolescence and early adulthood is a vitally important time for the achievement of peak bone mass, given that the most amount of bone an individual possesses is attained by about 30 years of age; this also corresponds to the timeframe across which most athletes have their competitive careers. For example, young competitive swimmers (notably females) may be prone to lower BMD and osteoporosis due to the nature of the sport being water borne and this providing less loading of the bones. Under these circumstances it may be prudent to promote some impact-based exercise routines (e.g., plyometrics and whole body resistance training) to strengthen bones.
Understanding the impact of various sports on the long-term bone health of athletes is not simple, since there is no comprehensive data relating to the number of ex-athletes from different sports suffering from osteopenia or osteoporosis in their older age. In addition, it is hard to know whether it is relevant to compare bone responses in athletes to normal population data, such as the t-score (which compares how much a person’s bone mass deviates from the bone mass of the average healthy 30-year-old adult) or the z score (which compares average bone mass to people of the same age and gender). In many cases, athletes are smaller (e.g., marathon runners and jockeys) or larger (e.g., rugby prop forwards) than the average individual, and so these general population comparisons might be misleading. Because of the specific nature of sports training and competition, it might be that, whilst the athlete has lower bone mass at the whole-body level, they have quite strong bones at specific skeletal sites.
Nutrition and bone health
The bone responds to diet and nutrition across the lifespan (Mitchell et al., 2015) but also acutely, with previous studies showing that bone (re) modelling marker concentrations are reduced with feeding when compared to fasting (Clowes et al., 2002). When individuals are fed, both the rates of bone resorption (loss) and bone formation (gain) decrease, although the decrease in the rate of bone resorption is greater (Walsh and Henriksen, 2010), which might be explained by the fact that feeding influences several hormones that can subsequently regulate bone turnover.
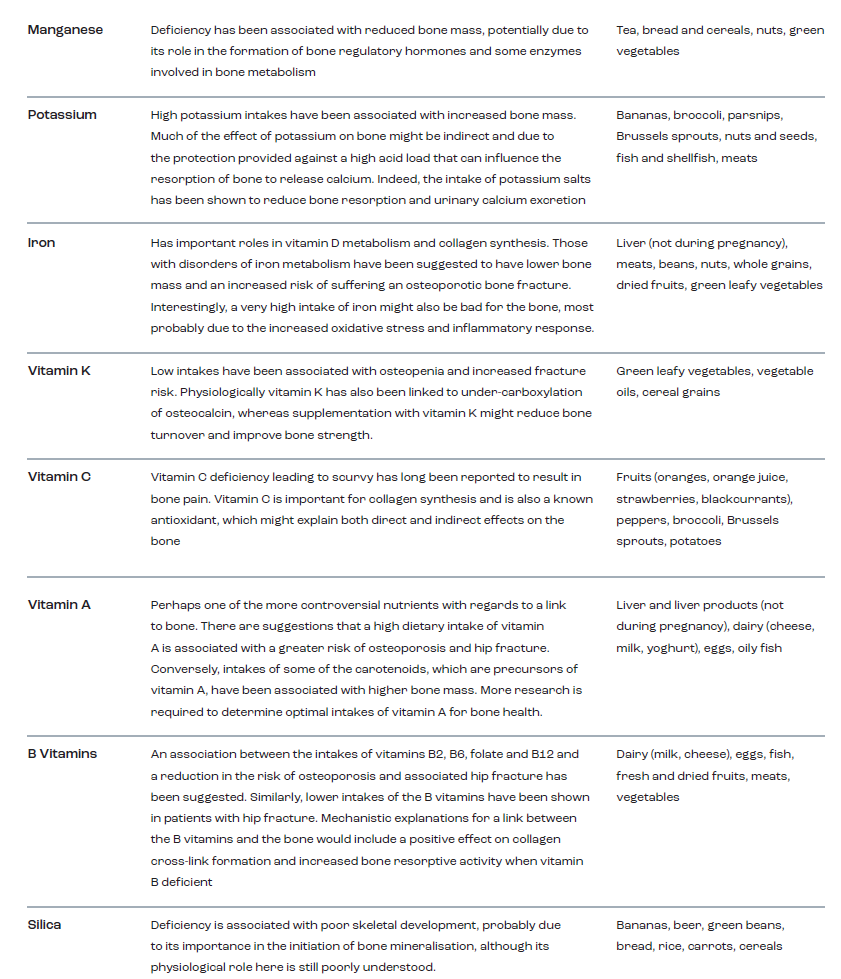
From a practical perspective, it is important to identify those nutrients and foods that best support the skeleton and, equally, to identify whether there are any dietary conditions that place athletes at increased risk of bone injury or reduced BMD and strength. In general terms, the dietary requirements to support the nutritional needs of bone are not likely to be any different for the athlete than for the general population. Some of these key nutrients are summarised in Table 1 (Sale & Elliott-Sale, 2019). Whilst much of the general dietary information around the impact of diet on musculoskeletal health has largely focused upon calcium, vitamin D, and protein, it is important to note that evidence exists to support the beneficial effects of other aspects of the diet. For example, vegetables provide key nutrients (e.g., potassium, iron, vitamin K, vitamin C, silica) essential for muscle function and bone health, both of which are key factors in the prevention of falls and fractures (for a review see Webster et al., in press).
Although there is reasonable information on some of the key nutrients supporting bone health and there are recommended daily intakes for these nutrients (for examples, see the guidelines from the European Food Safety Authority, National Health and Medical Research Council and the Institute of Medicine), it remains unclear what impact the strenuous exercise training performed by many athletes might have upon the requirement for these nutrients. It is possible, or even likely, that the required intakes of various nutrients are increased significantly in the athlete, particularly when referring to optimising the intake of these nutrients rather than simply trying to avoid a deficiency (Larson-Meyer et al., 2018). As such, some consultation with a performance nutritionist or sports dietitian is recommended to attain a quality dietary assessment related to bone health, which should consider the intakes of dairy, fish, fruits and vegetables (particularly of the green leafy kind), since these are the most cited sources of the main nutrients supporting bone health.
The athlete, as with members of the general population, will have food preferences and intolerances that might prevent or limit the intake of certain nutrients important for bone health. For example, the increased prevalence of plant-based dietary approaches in the general population is mirrored in the athlete population, making it important to consider the effect of such dietary practices on not only performance, but also health outcomes (including bone health). Diets high in fruits and vegetables are linked to improved health outcomes, although this might not be quite as straight forward in relation to bone, since animal-based foods (including dairy products) contain high amounts of the nutrients that relate to positive outcomes for bone health. As such, it is possible that complete absence of animal-based foods from the athlete’s diet could negatively impact bone health. Some recent epidemiological data support this notion, indicating poorer bone health in vegans and vegetarians, particularly in relation to a higher risk of fragility fractures (Thorpe et al., 2021; Tong et al., 2020; Webster et al., 2022). That said, there is only limited investigation of the possible impact of plant-based diets on bone related outcomes in athletes. Of some relevance is a prospective study conducted to examine incidence of stress fractures amongst Indian military recruits, which reported that the incidence of stress fracture injury was significantly greater in vegetarian recruits compared with non-vegetarian recruits (Dash and Kushwaha, 2012).
Some issues specific to the athlete
Although the general dietary requirements underpinning bone health are similar in the athlete to the general population, there are some dietary/nutritional challenges specific to the athlete, which may include low energy availability, low carbohydrate availability, protein intake, vitamin D intake and dermal calcium losses; although this list is by no means exhaustive.
Low energy availability
Changes in energy balance and energy availability have both been used to identify energy deficiency in athletes and athletic individuals, with energy balance being calculated as total energy expenditure minus dietary energy intake; and energy availability as dietary energy intake minus exercise energy expenditure, which is adjusted for fat free mass. Although similar, the two differ slightly, but importantly in this context in the fact that energy balance assumes that bodily systems are functioning normally. For example, the increased energy expenditure observed with strenuous exercise and training might suppress some bodily functions and so, at least in theory, individuals could be in energy balance, but still experience low energy availability (Papageorgiou et al., 2017).
The link between continuous/longer-term low energy availability and poorer bone health is best known for being described as part of the female (Nattiv et al., 2007) and male (Tenforde et al., 2016) athlete triads, and the relative energy deficiency in sport syndrome (Mountjoy et al., 2014). The investigation of this link is significantly hampered by the fact that it is difficult to accurately assess energy availability, particularly in the longer-term. For example, it could often be the case that energy intake is underestimated, whilst energy expenditure is overestimated. Additionally, in the long-term, it is also difficult to isolate the effects of low energy availability on bone from other nutritional (such as carbohydrate availability, discussed below) and non-nutritional (such as exercise factors, poor sleep, illnesses, and life/psychological stress) factors.
Whilst being in a very low energy availability state in the long-term is likely to be bad for bone health, it is less clear whether a quantitative level can be applied above which bone health is protected, even if this level falls below energy balance, which has often been suggested to be at 45 kcal·kgLBM-1·d-1. Ihle and Loucks (2004), in a short-term laboratory study, showed that bone formation (gain) was significantly reduced at an energy availability of 30 kcal·kgLBM-1·d-1, but that bone resorption (loss) remained unaffected.
Whilst this has been used to suggest a potential ‘threshold’ of energy availability to avoid the negative consequences on bone health and adverse alterations to hormone levels important to the female reproductive system, the original work was not designed for this purpose. Indeed, it is highly unlikely that this would provide a diagnostic endpoint, especially when considering a) the likely large individual differences in response to specific levels of energy availability and b) the significant difficulties in accurately measuring energy availability, particularly in athletes.
In addition to the magnitude of low energy availability, it also remains unclear whether there is a particular duration of low energy availability that negatively influences bone health. Taken together, this suggests significant difficulty in determining the direct impact of low energy availability on bone health that is intermittently applied or not continuously applied over the longer-term. Further research is clearly required in larger numbers of athletes and employing optimal methods to significantly improve our understanding of this area.
Low carbohydrate availability
It is becoming clear that many athletes, including endurance athletes, do not meet recommended carbohydrate intake patterns in competition (Sampson et al., 2023), and, even in training, many athletes seem to consume lower than ideal amounts of carbohydrate. Part of this might relate to the reported benefits of lower carbohydrate intakes for body composition or because it is suggested that it might help improve the endurance adaptations to training. In some cases, however, lower intakes of carbohydrate than would be recommended might not be deliberate and could relate to a lack of athlete knowledge / education about carbohydrate intake requirements and/or the foods required to meet recommendations. In addition to the possible direct effects of low carbohydrate availability on bone health, which will be discussed below, there might also be a concern that these dietary approaches could increase the risk of a low energy availability state.
Hammond et al. (2019) showed that consuming CHO before, during and after high-intensity interval running attenuates bone resorption (with no effects on bone formation); effects that were independent of energy availability. These findings are in line with prior studies showing that carbohydrate provision reduces the bone resorption response to acute exercise in athletes completing 8 days of overloaded endurance training (de Sousa et al., 2014), and that there was a post-exercise reduction in bone (re) modelling marker concentration with carbohydrate feeding during a 120-minute treadmill run in recreationally active individuals (Sale et al., 2015). As such, there is a small but developing body of work suggesting that carbohydrate feeding before, during or after exercise might be a useful way to modify the acute bone response to hard exercise and training, although further research is required to confirm this.
Although no long-term studies of athletes following a low carbohydrate diet are available in relation to bone health, evidence from shorter term studies with athletes following a low carbohydrate, high fat diet would seem to indicate altered bone metabolism. Heikura et al. (2020) studied the effects of a 3.5-week ketogenic low-carbohydrate, high-fat diet, and subsequent restoration of carbohydrate (CHO) feeding on bone (re)modelling marker concentrations in elite race walkers. They showed that the low carbohydrate, high fat diet increased fasting markers of bone resorption (loss) and decreased markers of bone formation (gain), with carbohydrate restoration in the diet returning bone resorption marker concentrations to pre-intervention levels. In a follow-on study from the same group (again in elite racewalkers), Fensham et al. (2022) showed that short-term carbohydrate restriction resulted in reduced resting and post-exercise bone formation marker concentrations. In addition, exercise in the carbohydrate restricted state resulted in increased in bone resorption marker concentrations. By contrast, no alterations to bone (re)modelling marker concentrations were shown when athletes maintained adequate carbohydrate intake.
Taken together, these results suggest that low carbohydrate availability might have a negative impact upon athlete bone health, should these acute and shorter-term effects continue over the longer-term. Clearly, longer term studies are required to determine the impact of low carbohydrate diets on bone mass and strength in athletes, although independent effects of low carbohydrate would be difficult to establish.
Protein intake
Both endurance and strength and power-based athletes are advised to consume more protein than is recommended for the general population (i.e. more than 0.8 g·kgBM-1·d-1), which presents an interesting issue in relation to bone health. It has been previously reported that higher animal protein intakes may have an adverse effect on bone health. The acid-ash hypothesis (Fenton et al., 2008) proposed that a higher intake of animal proteins create a significant challenge to the maintenance of acid-base balance and, to rectify this, the body increases the availability of alkaline minerals, such as calcium, by breaking down the bone to release the stored calcium. The excess calcium would be lost via excretion in the urine, and if continued over time, these processes would increase the rate of bone loss and cause a reduction in bone mass (Macdonald et al., 2005).
Whilst this hypothesis is plausible, it is now commonly recognised that protein intakes above the recommended amounts and in amounts recommended for athletes, even if containing high animal protein, do not present a significant risk for bone health (Dolan and Sale, 2018). Although not using optimal methods, Antonio et al. (2018) showed that bone mass was not affected by high-protein consumption, at ≥2.2 g·kgBM-1·d-1 over 6 months in exercise trained women. That said, under these circumstances, it would likely be prudent for athletes to make sure that their diet contains adequate calcium to reduce the potential for any calcium disturbances to impact upon bone health. Although not based upon optimal methods of dietary assessment, with only a single 24h dietary record being used for intake data, recent data from Bergamo et al. (2023) showed that higher protein and low calcium intakes were associated with lower bone mass.
Despite the acid-ash hypothesis, it is equally possible that higher protein intakes could be beneficial for the bone, either directly or indirectly. Protein forms an important part of the bones’ structure (Zimmerman et al., 2015), and protein ingestion increases the production of growth factors and hormones involved in the formation of bone (for a detailed review see Dolan and Sale, 2018). There are, however, limited long-term data on the effects of high protein intakes (particularly with higher animal protein intakes) on bone mass and strength in athletes.
Vitamin D
A direct relationship between serum vitamin D levels and bone health outcomes is relatively clear (Scientific Advisory Committee on Nutrition, 2016), and it is equally clear that several athlete groups are at risk of deficient or insufficient levels of circulating vitamin D (Owens et al., 2015). Given
the well-identified link between low vitamin D levels (serum 25-hydroxyvitamin D [25OHD] levels below 25 nmol·L-1) and bone, where it plays an important role in calcium and phosphorus regulation, it is highly likely that athletes deficient in vitamin D will be at a greater risk of low bone mass (Hollick, 2007), not to mention bone injuries. Maroon et al. (2015) reported that vitamin D levels were significantly lower in professional American Football players with at least one bone fracture when compared with no fractures. Although not athletes per se, female military recruits supplementing with 2000 mg calcium and 800 IU of vitamin D per day had
a 20% lower incidence of stress fracture injury than recruits supplemented with placebo (Lappe et al., 2008). In female runners, a greater intake of dairy products (including vitamin D, but also other nutrients important for bone) was associated with lower stress fracture rates (Nieves et al., 2010). In general, it remains clear that athletes should avoid vitamin D deficiency and insufficiency to protect their bone health.
Calcium losses and supplementation
Athletes who perform a significant volume of prolonged strenuous exercise could be at risk of losing enough calcium through sweating to result in a decline in serum calcium concentrations, although Kohrt et al. (2019) have suggested that dermal calcium losses are not likely to be a major cause of the increases in parathyroid hormone and bone resorption during shorter term exercise. That said, this study only examined the effects of 60 min of cycling at ~75% of peak, in cool (18°C) and warm (26°C) conditions to elicit differences in sweating, and it might be that dermal calcium losses in endurance and ultra-endurance events might be sufficient to cause a disruption to calcium homeostasis. Should this occur, it would increase parathyroid hormone secretion and promote the resorption of bone to release calcium into the circulation to defend the serum calcium level. Under these circumstances, should they occur, it is possible that providing athletes with some calcium before or during exercise might compensate for calcium losses and help to maintain serum calcium levels, negating the linked increase in secretion of parathyroid hormone and bone breakdown, as originally suggested by Barry et al. (2011).
Two main studies in athletes have been conducted to examine the effects of calcium supplementation on circulating parathyroid hormone and bone (re) modelling marker concentrations. Haakonssen et al. (2015) provided a calcium rich pre-exercise meal (~1350mg), which significantly reduced both parathyroid hormone and bone resorption responses to a subsequent 90-minute cycling bout in competitive female cyclists. Recent data from the same group (Lundy et al., 2023), showed that providing 1000mg of calcium (when compared to a low calcium intake at <10mg of calcium) prior to two rowing training sessions conducted on the same day, maintained serum calcium levels during exercise, which subsequently reduced post-exercise parathyroid hormone and bone resorption marker levels. These studies would suggest that there might be value for some athletes in considering their pre-exercise calcium intakes, but more research is required, particularly to determine the longer-term implications of this for bone health and/or injury risk.
Closing remarks and future perspectives
Research into the effects of nutrition on the bone health of athletes is still in its infancy, at least at a scale that is likely to make a significant difference to our knowledge base on the topic. In fact, there is still a lot that is not known in relation to both the development of bone stress injuries in different types of athletes and, even more so, about the implications of being an athlete for long-term bone health. As such, it is difficult to provide specific practical guidelines relating to the optimal dietary and nutrient intakes required to protect the bone and optimise positive adaptations.
It is safe to suggest that, where possible, athletes should consider a practitioner supported assessment of dietary intake to determine whether they are consuming adequate amounts of the key nutrients underpinning bone health, although, for the athlete, it is not yet possible to be clear on exactly what optimal intakes of these nutrients would be. The practitioner must, of course, work within the athletes’ dietary preferences and intolerances, which might necessitate the use of supplements or fortified foods.
It would stand to reason that most athletes should at least make sure they are not vitamin D deficient or insufficient and to achieve a serum vitamin D level of above 50 nmol·L-1 (20 ng·mL-1). Once again though, it is not possible from the evidence available to suggest a definitive target for serum vitamin D levels to prevent bone stress injuries. In addition to vitamin D, it is worth some athletes, particularly endurance and ultra-endurance athletes, considering dietary their calcium intake or supplementation prior to hard or prolonged exercise bouts. Finally, it is also important for athletes to be educated around energy and carbohydrate availability, such that periods of low energy or carbohydrate availability are periodised around essential aspects of the athletes training where the greatest performance benefit is likely to occur.
References
Antonio J, Ellerbroek A, Evans C, Silver T, Peacock CA. High protein consumption in trained women: bad to the bone? J Int Soc Sports Nutr. 2018; 15: 6.
Barry DW, Hansen KC, van Pelt RE, Witten M,Wolfe P, Kohrt WM. Acute calcium ingestion attenuates exercise-induced disruption of calcium homeostasis. Med Sci Sports Exerc.2011; 43(4): 617-623.
Bergamo RR, Páscoa MA, Hespanhol JE, de Moraes AM, Guerra-Júnior G. Positive association of lean mass and negative association of protein intake on bone mass and bone geometry of adolescent soccer players. Nutrition. 2023; 105: 111857.
Clowes JA, Hannon RA, Yap TS, Hoyle NR, Blumsohn A, Eastell R. Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone. 2002; 30(6): 886-890.
Dash N, Kushwaha A. Stress fractures-a prospective study amongst recruits. Med J Armed Forces India. 2012; 68(2):118-122.
de Sousa MV, Pereira RM, Fukui R, Caparbo VF, da Silva ME. Carbohydrate beverages attenuate bone resorption markers in elite runners. Metabolism. 2014; 63(12): 1536-1541.
Dolan E, Sale C. Protein and bone health across the lifespan. Proc Nutr Soc. 2018; 78(1): 45-55.
Fensham NC, Heikura IA, McKay AKA, Tee N, Ackerman KE, Burke LM. Short-Term Carbohydrate Restriction Impairs Bone Formation at Rest and During Prolonged Exercise to a Greater Degree than Low Energy Availability. J Bone Miner Res. 2022; 37(10): 1915-1925.
Fenton T, Eliasziw M, Lyon A, Tough SC, Hanley DA. Meta-analysis of the quantity of calcium excretion associated with the net acid excretion of the modern diet under the acid ash diet hypothesis. Am J Clin Nutr. 2008; 88: 1159–1166.
Fredericson M, Chew K, Ngo J, Cleek T, Kiratli J, Cobb K. Regional bone mineral density in male athletes: a comparison of soccer players, runners and controls. Br J Sports Med.2007; 41(10), 664-668.
Haakonssen EC, Ross ML, Knight EJ, Cato LE,Nana A, Wluka AE, et al. The effects of a calcium-rich pre-exercise meal on biomarkers of calcium homeostasis in competitive female cyclists: a randomised crossover trial. PLoS One. 2015; 10(5): e0123302.
Hammond KM, Sale C, Fraser W, Tang J, Shepherd SO, Strauss JA, Close GL, Cocks M, Louis J, Pugh J, Stewart C, Sharples AP, Morton JP. Post-exercise carbohydrate and energy availability induce independent effects on skeletal muscle cell signalling and bone turnover: implications for training adaptation. J Physiol. In press.
Heikura IA, Burke LM, Hawley JA, Ross ML, Garvican-Lewis L, Sharma AP, McKay AKA, Leckey JJ, Welvaert M, McCall L, Ackerman KE. A Short-Term Ketogenic Diet Impairs Markers of Bone Health in Response to Exercise. Front Endocrinol. 2020; 10: 880.
Hoenig T, Ackerman KE, Beck BR, Bouxsein ML, Burr DB, Hollander K, Popp KL, Rolvien T, Tenforde AS, Warden SJ. Bone stress injuries. Nat Rev Dis Primers. 2022; 8(1): 26.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357(3): 266-281.
Hendrickx G, Boudin E, Van Hul W. A look behind the scenes: the risk and pathogenesis of primary osteoporosis. Nat Rev Rheumatol.
2015; 11(8): 462-74.
Ihle R, Loucks AB. Dose-response relationships between energy availability and bone turnover in young exercising women. J Bone Miner Res. 2004; 19(8): 1231-1240.
Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D: Institute of Medicine of the National Academies, 2010.
Kohrt WM, Wolfe P, Sherk VD, Wherry SJ, Wellington T, Melanson EL, Swanson CM, Weaver CM, Boxer RS. Dermal Calcium Loss Is Not the Primary Determinant of Parathyroid Hormone Secretion during Exercise. Med Sci Sports Exerc. 2019; 51(10), 2117-2124.
Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008; 23(5): 741–749.
Larson-Meyer ED, Woolf K, Burke L. Assessment of nutrient status in athletes and the need for supplementation. Int J Sports Nutr Exerc Metab. 2018; 28, 139-158.
Lundy B, McKay AKA, Fensham NC, Tee N, Anderson B, Morabito A, Ross MLR, Sim M, Ackerman KE, Burke LM. The Impact of Acute Calcium Intake on Bone Turnover Markers during a Training Day in Elite Male Rowers. Med Sci Sports Exerc.
2023; 55(1): 55-65.
Macdonald HM, New SA, Fraser WD, Campbell MK, Reid DM. Low dietary potassium intakes and high dietary estimates of net endogenous acid production are associated with low bone mineral density in premenopausal women and increased markers of bone resorption in postmenopausal women. Am J Clin Nutr. 2005; 81: 923–933.
Maroon JC, Mathyssek CM, Bost JW, Amos A, Winkelman R, Yates AP, Duca MA, Norwig JA. Vitamin D profile in National Football League players. Am J Sports Med. 2015; 43(5): 1241-1245.
McBryde, AM. Stress fractures in runners. Clin Sports Med. 1985; 4(4), 737-752.
Mitchell PJ, Cooper C, Dawson-Hughes B, Gordon CM, Rizzoli R. Life-course approach to nutrition. Osteoporos Int. 2015; 26: 2723-2742.
Mountjoy M, Sundgot-Borgen J, Burke LM, Carter S, Constantini N, Lebrun C, et al. The IOC consensus statement: beyond the Female Athlete Triad--Relative Energy Deficiency in Sport (RED-S). Br JSports Med. 2014; 48(7): 491-497.
National Institute for Health and Clinical Excellence. Osteoporosis fragility fracture risk - Costing report. 2012.
Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP, et al. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007; 39(10): 1867-1882.
Nieves JW, Melsop K, Curtis M, Kelsey JL, Bachrach LK, Greendale G, Sowers MF, Sainani KL. Nutritional factors that influence change in bone density and stress fracture risk among young female cross-country runners. PM R. 2010; 2(8): 740-750.
Owens DJ, Fraser WD, Close GL. Vitamin D and the athlete: emerging insights. Eur J Sport Sci.2015; 15(1): 73-84.
Papageorgiou M, Elliott-Sale KJ, Parsons A, Tang JCY, Greeves JP, Fraser WD, et al. Effects of reduced energy availability on bone metabolism in women and men. Bone. 2017; 105: 191-199.
Ranson CA, Burnett AF, Kerslake RW. Injuries to the lower back in elite fast bowlers: acute stress changes on MRI predict stress fracture. J Bone Joint Surg Br. 2010; 92(12), 1664-1668.
Sale C, Elliott-Sale KJ. Nutrition and Athlete Bone Health. Sports Med. 2019; 49(S2): 139-151.
Sale C, Varley I, Jones TW, James RM, Tang JC, Fraser WD, et al. Effect of carbohydrate feeding on the bone metabolic response to running. J Appl Physiol. 2015; 119(7): 824-830.
Sampson G, Morton JP, Areta JL. Mind the gap: limited knowledge of carbohydrate guidelines for competition in an international cohort of endurance athletes. Journal of Nutritional Science.2023; 12(68): 1-16.
Santos L, Elliott-Sale KJ, Sale C. Exercise and bone health across the lifespan. Biogerontology. 2017; 18(6), 931-946.
Scientific Advisory Committee on Nutrition. Vitamin D and Health. 2016; https://www.gov.uk/government/groups/scientific-advisory-committee-on-nutrition.Tenforde AS, Barrack MT, Nattiv A, Fredericson M. Parallels with the female athlete triad in male athletes. Sports Med. 2016; 46(2), 171-182.
Thorpe DL, Beeson WL, Knutsen R, Fraser GE, Knutsen SF. Dietary patterns and hip fracture in the Adventist Health Study 2: combined vitamin D and calcium supplementation mitigate increased hip fracture risk among vegans. Am J Clin Nutr.
2021; 114(2): 488-495.
Tong TYN, Appleby PN, Armstrong MEG, Fensom GK, Knuppel A, Papier K, Perez-Cornago A, Travis RC, Key TJ. Vegetarian and vegan diets and risks of total and site-specific fractures: results from the prospective EPIC-Oxford study. BMC Med.
2020; 18(1): 353.
Walsh JS, Henriksen DB. Feeding and bone. Arch Biochem Biophys. 2010; 503(1): 11-19.
Webster J, Dalla Via J, Langley C, Smith C, Sale C, Sim M. Nutritional strategies to optimise musculoskeletal health for fall and fracture prevention: Looking beyond calcium, vitamin D and protein. Bone Reports. In press.
Webster J, Greenwood DC, Cade JE. Risk of hip fracture in meat-eaters, pescatarians, and vegetarians: results from the UK Women’s Cohort Study. BMC Med. 2022; 20(1): 275.
Zimmerman E, Busse B, Ritchie R. The fracture mechanics of human bone: influence of disease and treatment. Bonekey Rep. 2015; 4: 743.









